A huge amount of the technology and comforts that we have in our world just wouldn’t exist if it wasn’t for what we’ve learned about the way heat behaves. So keep cool and use this excellent series to teach your students everything that they need to know about heat, including its effect on things and how it transfers from one thing to another.
In Episode 5, Heat Transfer, we look at the three ways that heat energy can transfer from one thing to another: conduction; convection; and radiation. A heat source is useless if heat energy can’t transfer from the heat source to whatever you want heated. Understanding heat transfer is essential if you want to cook good food and if you want to stay warm when it’s cold and stay cool when it’s hot.
A 4-minute excerpt followed by a 1 minute summary.
 The Episode 5 Question Sheet for Students:
The Episode 5 Question Sheet for Students:
![]() The PDF version.
The PDF version. ![]()
Google The Google Doc version. Google
Get the answers.
![]() If you have ClickView, watch the whole episode here.
If you have ClickView, watch the whole episode here.
![]() If you have Learn360, watch the whole episode here.
If you have Learn360, watch the whole episode here.
![]() If you have Films on Demand, watch the whole episode here.
If you have Films on Demand, watch the whole episode here.
![]() If you have Classroom Video, watch the whole episode here.
If you have Classroom Video, watch the whole episode here.
![]() Most of our videos are also available on SAFARI Montage. Just log in and do a quick search.
Most of our videos are also available on SAFARI Montage. Just log in and do a quick search.
![]() Don’t have any of the above? Rent or buy the Shedding Light series and/or individual programs from our Vimeo page!!
Don’t have any of the above? Rent or buy the Shedding Light series and/or individual programs from our Vimeo page!!
The Transcript (which can be used as a textbook)
Contents:
Part A: Introduction. There are three ways that heat can transfer from one thing to another.
Part B: Conduction: If you heat one end of a metal rod, the other end will also (after a while) get hot. Heat energy can transfer through things, but it can transfer through some things more easily than through others. Heat doesn’t transfer all that quickly through food, and so we’ll show you the right way and the wrong way to cook a leg of lamb.
Part C: Convection: It’s all about the currents. Hot air gets around and it carries heat energy with it. But beware of gas ovens where there’s more hot air at the top than there is at the bottom!
Part D: Radiation: How does heat energy get from the sun to the Earth? How does heat energy get from the hot barbecue coals to the meat being barbecued above it? And why are most of the buildings on the beautiful island of Santorini painted white?
Part A: Introduction
 Hello and welcome. In this episode of the Shedding Light on Heat series, we’re going to look at heat transfer, that is, how heat energy transfers from one thing to another.
Hello and welcome. In this episode of the Shedding Light on Heat series, we’re going to look at heat transfer, that is, how heat energy transfers from one thing to another.
For example, fire is a hugely important heat source that is used to heat things up, but there are a number of different ways that heat energy can transfer from a fire to whatever you want heated.
 In fact, there are three different ways that heat energy can transfer from one thing to another: conduction, convection, and radiation.
In fact, there are three different ways that heat energy can transfer from one thing to another: conduction, convection, and radiation.
We’ll begin by looking at conduction.
Part B: Conduction
 When you heat water in a pot, heat energy from the flame has to get into the water, but how does it get into the water when the metal that the pot is made of is in the way? Well, the heat transfers by conducting through the metal.
When you heat water in a pot, heat energy from the flame has to get into the water, but how does it get into the water when the metal that the pot is made of is in the way? Well, the heat transfers by conducting through the metal.
Conduction is the transfer of heat energy through a substance without any movement of the substance itself.
 Here I’ve set up a copper rod on a retort stand and I’m heating the rod at one end. Every 5 cm I’ve stuck a nail to the copper with a bit of Vaseline. The copper is being heated only at one end but the heat energy is conducting through it. As the heat conducts through the metal, the metal gets hotter and hotter one small section at a time. The Vaseline melts when the part of the rod that it’s sticking to gets hot and so the nails drop off one by one.
Here I’ve set up a copper rod on a retort stand and I’m heating the rod at one end. Every 5 cm I’ve stuck a nail to the copper with a bit of Vaseline. The copper is being heated only at one end but the heat energy is conducting through it. As the heat conducts through the metal, the metal gets hotter and hotter one small section at a time. The Vaseline melts when the part of the rod that it’s sticking to gets hot and so the nails drop off one by one.
 So, the heat energy doesn’t just conduct through the metal. It also conducts from the hot metal into the cooler Vaseline because they’re in direct contact with each other.
So, the heat energy doesn’t just conduct through the metal. It also conducts from the hot metal into the cooler Vaseline because they’re in direct contact with each other.
Metals are very good conductors of heat. It took only about 6 minutes for the heat to conduct right through.
Everything conducts heat but some things are good conductors and some things are poor conductors. Really poor conductors are called insulators.
 Glass is a poor conductor of heat energy. This is the same set up as the previous demonstration, but I’ve replaced the copper rod with a glass rod. It took nearly a minute and a half for the first nail to fall, 5 minutes for the second, 12 minutes for the third, and when we stopped the experiment 20 minutes later, the last two nails were still hanging on the glass.
Glass is a poor conductor of heat energy. This is the same set up as the previous demonstration, but I’ve replaced the copper rod with a glass rod. It took nearly a minute and a half for the first nail to fall, 5 minutes for the second, 12 minutes for the third, and when we stopped the experiment 20 minutes later, the last two nails were still hanging on the glass.
It turns out that all metals, like copper, tin, and iron are good conductors of heat, while plastics, rubber, wood, and fabrics made of, say, wool and cotton are poor conductors of heat. We typically call poor conductors insulators, but just remember that everything conducts heat at least a little.
Conduction, like so many other things relating to heat can be explained once again by the Kinetic Theory. A copper rod is made up of copper atoms which are all vibrating in their fixed positions. If we heat the atoms one end of the metal rod, those atoms start to vibrate faster and faster. These fast-vibrating atoms kind of crash into the atoms that are next to them and cause them to start vibrating faster as well. In turn, these hot atoms, that aren’t actually in the flame, crash into the atoms next to them and they start to vibrate faster as well. The process continues and the heat energy transfers, that is, it conducts along the metal and into anything the metal is touching, like the water in a pot.
 So when you boil water, the heat energy conducts through the metal of the pot and then also conducts into the water that’s in direct contact with the pot.
So when you boil water, the heat energy conducts through the metal of the pot and then also conducts into the water that’s in direct contact with the pot.
Conduction occurs whenever any two things that are at different temperatures are in contact with each other. If I go to pull this very hot baking tray out of the oven with my bare hands, a huge amount of heat energy will conduct from the metal into my hands and my skin will burn. Oven gloves are made of fabric which is a very poor conductor of heat, so very little heat conducts through them, allowing me to pick up the baking tray without getting burned.
 Frying pans are usually made of metal because metals are good conductors, while the handles are generally made of hard plastics that are insulators. Heat doesn’t conduct through the plastic handle and so the end of it remains cool enough to hold.
Frying pans are usually made of metal because metals are good conductors, while the handles are generally made of hard plastics that are insulators. Heat doesn’t conduct through the plastic handle and so the end of it remains cool enough to hold.
Whenever you cook food, you have to cook it in such a way that allows the heat to conduct all the way through the food so that all of it cooks properly.
Meat, like most foods, is a poor conductor of heat and so, when you roast it, it takes a long time for the heat to conduct through it to the middle. So the meat has to be cooked at the right temperature and for the right amount of time to make sure that the inside has time to cook but that the outside doesn’t burn before the inside cooks.
 In this experiment, I roasted this leg of lamb at a temperature of 250°C for about 1 hour. When I removed it from the oven, it was golden brown and crispy and looked fairly well cooked. However, when I cut the meat open, we can see that very little heat energy conducted through to the middle of the meat and so the middle was quite raw. The meat cooked only to a depth of a few centimetres. If I had kept it in the oven for longer, the inside would have cooked eventually, but by the time it did, the outside would have been badly burned.
In this experiment, I roasted this leg of lamb at a temperature of 250°C for about 1 hour. When I removed it from the oven, it was golden brown and crispy and looked fairly well cooked. However, when I cut the meat open, we can see that very little heat energy conducted through to the middle of the meat and so the middle was quite raw. The meat cooked only to a depth of a few centimetres. If I had kept it in the oven for longer, the inside would have cooked eventually, but by the time it did, the outside would have been badly burned.
 Now this leg of lamb was roasted at a much cooler temperature of 180°C for about 4 hours. Did it make any difference to how the meat has cooked? Let’s have a look? It looked pretty good on the outside but the heat also conducted through to the middle to cook the inside as well. Whenever you cook anything, the heat source has to be hot enough to cook the food of course, but not so hot that the outside cooks before the heat has a chance to conduct into the middle of the food to cook that as well.
Now this leg of lamb was roasted at a much cooler temperature of 180°C for about 4 hours. Did it make any difference to how the meat has cooked? Let’s have a look? It looked pretty good on the outside but the heat also conducted through to the middle to cook the inside as well. Whenever you cook anything, the heat source has to be hot enough to cook the food of course, but not so hot that the outside cooks before the heat has a chance to conduct into the middle of the food to cook that as well.
So, temperature and time are both important. By the way, after we filmed the first, half-cooked leg of lamb, I actually cut the cooked meat off and then placed the rest back into the oven, at a lower temperature of course. No point wasting it!
 Now in cooking, the heat travels from the outside inwards. In cold weather though, the heat travels from the inside of our bodies outwards. Remember heat energy always travels from hotter objects towards colder objects. In cold weather, heat energy can conduct from our bodies into the cold air that surrounds us and so we feel cold.
Now in cooking, the heat travels from the outside inwards. In cold weather though, the heat travels from the inside of our bodies outwards. Remember heat energy always travels from hotter objects towards colder objects. In cold weather, heat energy can conduct from our bodies into the cold air that surrounds us and so we feel cold.
Clothes are made of fabrics that are very good insulators and so they stop a lot of the heat escaping from our bodies into the air. The clothes don’t provide heat energy, it’s our own bodies that produce the heat energy, but clothes simply reduce the rate of heat loss from our bodies. Basically they trap the heat energy and stop it from escaping too quickly.
 So, which substances are the best conductors and which are the best insulators? Scientists measure what’s called the Thermal Conductivity of different substances by placing them near a heat source and measuring how much energy passes through them.
So, which substances are the best conductors and which are the best insulators? Scientists measure what’s called the Thermal Conductivity of different substances by placing them near a heat source and measuring how much energy passes through them.
The Thermal Conductivity is a measure of how much energy in Joules flows through a substance per second taking into account the thickness of the substance and the temperature difference between one side and the other.
 Here are the thermal conductivities of some common materials. As you can see, metals are very good thermal conductors. Water is a fairly poor thermal conductor and so foods, which typically contain lots of water of course, are also fairly poor conductors. We saw that earlier with the legs of lamb I cooked. Meat is about 75% water.
Here are the thermal conductivities of some common materials. As you can see, metals are very good thermal conductors. Water is a fairly poor thermal conductor and so foods, which typically contain lots of water of course, are also fairly poor conductors. We saw that earlier with the legs of lamb I cooked. Meat is about 75% water.
Building materials like rock and stone, concrete, bricks, plaster and wood are also fairly poor conductors, but since over a whole day lots of heat can pass through them, buildings typically use additional insulation materials to reduce heat loss from the building in cold weather and to reduce heat gain from the outside in the summer. We’ll look at these in a little more detail later.
Materials used to makes clothes like wool, cotton, polyester, and feathers (which are… bird clothes) are all excellent insulators.
All clothing materials are not just insulators in their own right, but they trap air between the fibres and between the layers and air is an excellent insulator.
As you can see air has an extremely low thermal conductivity and, as long as it doesn’t blow around, does a great job of trapping heat. Out here it might be freezing cold, but just a few centimetres away in here, it’s toasty warm.
 Of course, it’s not just the type of material that counts, it’s also the thickness of the material. Not much heat can conduct through one blanket, but even less heat conducts through two blankets.
Of course, it’s not just the type of material that counts, it’s also the thickness of the material. Not much heat can conduct through one blanket, but even less heat conducts through two blankets.
Solids are generally much better conductors than liquids and gases because of the fact that the atoms in solids are closer together. So if one side of a solid is heated the vibrations transfer much more quickly from atom to atom.  However, even though liquids and gases are not good conductors, since the atoms are free to move around they can form currents that carry heat energy from one place to another. This process of heat transfer is called convection. Let’s take a closer look.
However, even though liquids and gases are not good conductors, since the atoms are free to move around they can form currents that carry heat energy from one place to another. This process of heat transfer is called convection. Let’s take a closer look.
Part C: Convection
This small electric heater has a coiled loop of wire inside it that gets really hot when electricity passes through it. It also has a fan. The heater is designed to heat small rooms like a bedroom.
I’m standing about 2 metres away from the heater and it’s keeping me warm, but how does the heat being generated way over there get to me way over here? The heat doesn’t conduct, because air is not a good conductor. Well, quite simply, the air passing through the heater and heating up over there gets blown over towards me thanks to the fan that’s in the heater and carries the heat energy with it.
 Convection is the name given to the process by which heat energy is transferred on currents of air or in fact any liquid or gas. The word convection comes from the word convey which means to transport something (from one place to another, of course).
Convection is the name given to the process by which heat energy is transferred on currents of air or in fact any liquid or gas. The word convection comes from the word convey which means to transport something (from one place to another, of course).
The heat from the heater is being transferred to me by convection on the air that is being blown over towards me.
The word convection is also related to the word conveyor, as in “conveyor belt”. A conveyor belt continuously moves things from one place to another (and it’s also related to the word convoy, which is a group of ships or other vehicles that move together from one place to another).
 In a room, the air is kind of like a conveyor belt that carries the heat from the heater to the rest of the room. The warm air warms up whatever’s in the room and loses some of its warmth in the process. It then returns back to the heater and is reheated and the cycle repeats. The air current carrying the warm air is called a convection current.
In a room, the air is kind of like a conveyor belt that carries the heat from the heater to the rest of the room. The warm air warms up whatever’s in the room and loses some of its warmth in the process. It then returns back to the heater and is reheated and the cycle repeats. The air current carrying the warm air is called a convection current.
 Inside this central-heating unit under this house, natural gas is being burned to warm up the air being pumped through the unit. A large fan blows the warm air through these insulated air ducts and it comes out of these vents. The warm air then heats up the room. Once again, the heat is transferred by convection from the unit to the rooms of the house.
Inside this central-heating unit under this house, natural gas is being burned to warm up the air being pumped through the unit. A large fan blows the warm air through these insulated air ducts and it comes out of these vents. The warm air then heats up the room. Once again, the heat is transferred by convection from the unit to the rooms of the house.
Of course you can’t just pump air into a room from the outside, because the air pressure in the room will increase too much. Every central-heating system has at least one return air duct so that air can also return to the central heating unit and be reheated. The inlet for the return air duct is typically placed away from the warm-air vents and so the returning air is typically a little cooler that the incoming air. The convection currents carry the heat energy of the fire in the one and only central-heating unit through the air ducts to as many rooms as you want.
 The air ducts are insulated of course to stop the heat escaping.
The air ducts are insulated of course to stop the heat escaping.
Convection is also responsible for the heating up and the cooking of this gnocchi. Gnocchi, by the way, is a bit like pasta but it’s usually made from potatoes. But anyway…
The gas flame is heating the metal pot and heat energy transfers by conduction through the metal and then also by conduction from the metal into the water at the bottom of the pot because they’re in direct contact. We’ve already looked at conduction.
 But, how does the heat then transfer from the water at the bottom of the pot to the water at the top of the pot? Water is not really a good conductor of heat.
But, how does the heat then transfer from the water at the bottom of the pot to the water at the top of the pot? Water is not really a good conductor of heat.
Well, the hot water actually rises from the bottom and makes contact with the gnocchi floating at the top. The heat energy is transferred by a current of hot water. This is convection at work.
Convection and conduction typically go together. In a heater, the air is heated by conduction when it comes into direct contact with the coil of wire but then the warm air moves over to, say, a person in the room, that’s convection, and then heat energy transfers to the person by conduction when the warm air actually comes into contact with the person.
 Let me illustrate in a way that allows you to see convection. This large beaker is being heated mostly on the left side. The warm water on that side rises but then cools as it gets to the top. The slightly cooler water then sinks and the process repeats continuously. The noodles allow us to see the current. So, warm water rises and cool water sinks, and the current that forms when this happens is called a convection current.
Let me illustrate in a way that allows you to see convection. This large beaker is being heated mostly on the left side. The warm water on that side rises but then cools as it gets to the top. The slightly cooler water then sinks and the process repeats continuously. The noodles allow us to see the current. So, warm water rises and cool water sinks, and the current that forms when this happens is called a convection current.
As a result of convection currents, the heat energy passing into the water gets distributed throughout the water.
 Now when the water being heated here at the bottom left turns to steam, the rising bubbles of steam also contribute to the convection current that forms. However, warm water rises and cool water sinks even if there are no bubbles.
Now when the water being heated here at the bottom left turns to steam, the rising bubbles of steam also contribute to the convection current that forms. However, warm water rises and cool water sinks even if there are no bubbles.
Let me demonstrate. Here I’ve heated some water in a small beaker. I can pour some food dye into the hot water and then cover it with foil which I’ll keep in place with a rubber band. Once I’ve clamped the small beaker in place, I can then lower it into a large beaker filled with cold water. When I punch a hole in the foil with a nail attached to a clamp, the warm water comes out and immediately rises. As I said warm water rises (if it’s surrounded by cold water of course).
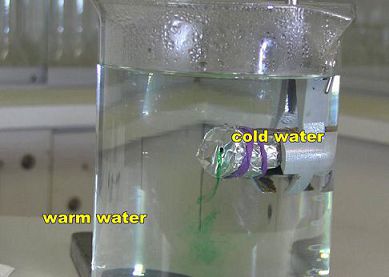 If I now fill the small beaker with ice-cold water and then place it into a beaker of hot water, then when I punch a hole in the foil, the cold water comes out of the little beaker and sinks. Cool water sinks (if it’s surrounded by warmer water of course). So in any situation where you have a body of water that doesn’t have an even temperature, the warmer water will rise and the cooler water will sink.
If I now fill the small beaker with ice-cold water and then place it into a beaker of hot water, then when I punch a hole in the foil, the cold water comes out of the little beaker and sinks. Cool water sinks (if it’s surrounded by warmer water of course). So in any situation where you have a body of water that doesn’t have an even temperature, the warmer water will rise and the cooler water will sink.
 Now warm air rises as well. This Bunsen burner flame is warming the air around it which then rises. It’s hard to tell of course, because we can’t see air, but if I hold a freezer bag over the flame and then let it go, the fact that warm air rises becomes obvious.
Now warm air rises as well. This Bunsen burner flame is warming the air around it which then rises. It’s hard to tell of course, because we can’t see air, but if I hold a freezer bag over the flame and then let it go, the fact that warm air rises becomes obvious.
Warm air rises up from a fireplace and of course carries the smoke, which we can see, with it.
This clever little experiment will also show us a convection current of air. If I cut a spiral out of a piece of paper, stick a pin through the top and then hold the spiral over a hot hot plate, then the warm air rising from the hot plate, which we can’t see of course, pushes on the spiral and makes it spin. The pin allows me to hold the spiral up but allows the spiral to turn.
Now heaters like theses ones actually blow hot air around with a fan, but here the air is rising on its own and here the heated water is also rising on its own, without being forced to. What causes warm water and warm air to rise? It comes down to the fact that warm water is less dense than cool water and likewise warm air is less dense than cool air.
The density of a substance is its mass per cubic centimetre. You can calculate the density of a substance by using the simple equation density = mass/volume.
| Temp (°C) |
Density pure water (g/cm3) |
| 0 (solid) | 0.9150 |
| 0 (liquid) | 0.9999 |
| 4 | 1.0000 |
| 20 | 0.9982 |
| 40 | 0.9923 |
| 60 | 0.9832 |
| 80 | 0.9718 |
| 100 (liquid) | 0.9584 |
The cubes here all have side lengths of 2.5 cm, so they each have a volume of 15.625 cm3.
The wooden cube has a mass of 12.1 grams so with a volume 15.625 cm3, its density is about 0.77 g/cm3. The aluminium cube has a density of about 2.7 g/cm3, while the zinc cube has a density of about 7.1 g/cm3.
Water’s density is about 1 g/cm3. Anything with a density less than that of water floats, while anything with a density greater than that of water sinks.
 Now let’s get back to the question of why warm water rises and cool water sinks. The density of water is about 1 g/cm3, but its density changes depending on its temperature. As we saw in our last episode, when water is heated, it expands. The mass doesn’t change when you heat something up but its volume does, which means that its density also does.
Now let’s get back to the question of why warm water rises and cool water sinks. The density of water is about 1 g/cm3, but its density changes depending on its temperature. As we saw in our last episode, when water is heated, it expands. The mass doesn’t change when you heat something up but its volume does, which means that its density also does.
At a temperature of 4°C, 1 gram of water has a volume of exactly 1.00 cm3. This was actually the original definition of one gram; one gram was defined as the mass of 1 cm3 of water at 4°C. At 20°C, one gram of water has a volume of 1.0018 cm3 (because of expansion), and at 100°C 1 g of water has a volume of 1.0434 cm3. The density of 4°C water is therefore exactly 1 g/cm3, of 20°C water is 0.9982 g/cm3 and of 100°C water is only 0.9584 g/cm3.
 Now remember, a denser liquid will sink below a less dense liquid.
Now remember, a denser liquid will sink below a less dense liquid.
So if you’ve got water being heated, the warmer water, being less dense, will rise to the top because it will literally float on top of the cooler water and the cooler water being more dense will therefore literally sink below the warmer water. However, the water that rises to the top moves away from the heat source and cools down so it sinks again, while at the same time the water that has sunk heats up and then rises. The flow of water is called, as I said earlier, a convection current.
 The same goes for air. Warm air is less dense than cool air so warm air rises and cool air sinks. The warm air moving upwards here is literally floating upwards on the cooler air that surrounds it and of course the cooler air is sinking beneath the warmer air.
The same goes for air. Warm air is less dense than cool air so warm air rises and cool air sinks. The warm air moving upwards here is literally floating upwards on the cooler air that surrounds it and of course the cooler air is sinking beneath the warmer air.
 Hot air balloons use this principle to float up into the air. Hot air balloons are less dense than the air around them and so they float upwards.
Hot air balloons use this principle to float up into the air. Hot air balloons are less dense than the air around them and so they float upwards.
The fact that warm air rises and that cool air sinks, means that the air at the top of a room can often be quite a lot warmer than the air at the bottom of a room. We heated this room, which was initially at 14°C, with an electric heater, and found that after an hour, the temperature near the floor was 18°C, near the middle was 22°C and near the ceiling was 24°C.

 The warm air from the heater rises upwards and tends to stay there.
The warm air from the heater rises upwards and tends to stay there.
Unfortunately, plaster, the material that many (if not most) ceilings are made of, is not a brilliant insulator. A lot of heat energy can conduct through the thin plaster ceiling into the cold ceiling cavity above it and so your heater is less effective because your heat is escaping and of course you waste money. Insulation materials like these insulation batts are made of glass or plastic fibres that trap millions of tiny pockets of air. The fibres themselves are good insulators but combined with the tiny air pockets the batts are excellent insulators. Installing insulation batts in your ceiling cavity greatly reduces the amount of energy escaping through the ceiling so a room will warm up quicker and it will stay warmer for longer.
In summer, the insulation reduces the heat coming into the room from the ceiling cavity which gets hot when the sun beats down on it. In Australia and I’m guessing in many countries, there are laws that specify how much insulation needs to be included in new buildings.
The fact that warm air rises and cool air sinks also means that the top of an oven will be 10, 20 or even 30°C warmer than the air at the bottom of the oven. I’m going to cook these biscuits, placing one tray at the top of the oven and the other tray near the bottom of the oven.
 The biscuits were baked for about 15 minutes. Now even though the oven was set to 170°C, it’s pretty obvious that the biscuits that were in the hotter air at the top of the oven have browned far more than the biscuits that were in the cooler air at the bottom of the oven. Clearly, the top of the oven is warmer than the bottom of the oven and the oven’s temperature dial is only a guide.
The biscuits were baked for about 15 minutes. Now even though the oven was set to 170°C, it’s pretty obvious that the biscuits that were in the hotter air at the top of the oven have browned far more than the biscuits that were in the cooler air at the bottom of the oven. Clearly, the top of the oven is warmer than the bottom of the oven and the oven’s temperature dial is only a guide.
 Using an infrared thermometer, we found that the metal near the top was about 160°C, while the metal near the bottom was about 140°C, although these were the temperatures after the door was opened. As soon as the door is opened, the oven cools down pretty quickly as warm air rushes up and out while cool air rushes in.
Using an infrared thermometer, we found that the metal near the top was about 160°C, while the metal near the bottom was about 140°C, although these were the temperatures after the door was opened. As soon as the door is opened, the oven cools down pretty quickly as warm air rushes up and out while cool air rushes in.
Many ovens, including this one, have built in fans that blow the hot air around the oven and this leads to a more even temperature. They’re called fan-forced convection ovens. I repeated the same experiment as before, but with the fan on, and after about 15 minutes found that the biscuits looked much the same. Clearly the air temperature at the top was pretty much the same as it was at the bottom.
Turning the fan on doesn’t just even out the temperature, it also tends to cook the food faster because the food is continuously being blasted by hot air. It also dries the food out a little more which crisps up the food, especially meat. However, sometimes you want the food to cook more slowly, so leaving the fan off might be better.
When warm air is actually blown by a fan like what this heater is doing, it’s often called “forced convection”, but when it just naturally rises on its own it’s simply called convection.
Now, whenever something is cooked by contact with hot air in an oven, it’s called roasting or baking.
However, this griller isn’t grilling the cheese here by heating the air near the cheese. Warm air moves upwards not downwards. No, a griller grills using heat radiation which is also called radiant heat. We’ve seen that heat energy can transfer from a hot object to a cold one by conduction and by convection. We now need to look at heat transfer by radiation.
Part D: Heat Radiation (or Radiant Heat)
 We all know that the sun warms our planet, but how does heat energy transfer from the sun to the Earth?
We all know that the sun warms our planet, but how does heat energy transfer from the sun to the Earth?
It can’t conduct because in space there’s nothing there through which the heat can conduct. The space between the sun and the Earth is well empty space. The atmosphere of the Earth extends only about 100 km or so from the surface, but about ¾ of the air is within 10 km of the surface. For the same reason, the heat energy can’t be transferred by convection either. There’s no air or anything else in space to form currents.
Heat energy is transferred from the sun to the Earth by what are called electromagnetic waves. The visible light that we can see coming from the sun is actually made of electromagnetic waves.
But this visible light is not the only type of light that exists. Visible light forms only a very small part of what is called the electromagnetic spectrum.
The electromagnetic spectrum consists of radio waves, microwaves, infrared light, visible light, Ultraviolet (or UV) light, X-rays, and gamma rays. They’re all really the same type of wave, but they are separated into groups just for convenience, based mainly on their wavelengths. For example, the wavelength of blue light is about 400 billionths of a metre and that of red light is about 700 billionths of a metre. The wavelengths of infrared waves varies from about 700 billionths of a metre up to about 1 mm.
 So what are these electromagnetic waves? Well, you need a bit of imagination but let me explain it this way. You’re all no doubt familiar with magnets. Surrounding every magnet is what’s called a magnetic field. We can’t see it, but we know that it’s there because nails for example within a magnetic field experience a force.
So what are these electromagnetic waves? Well, you need a bit of imagination but let me explain it this way. You’re all no doubt familiar with magnets. Surrounding every magnet is what’s called a magnetic field. We can’t see it, but we know that it’s there because nails for example within a magnetic field experience a force.
 You’re probably also familiar with static electricity. When you rub say a balloon on fabric, you can use it to attract little pieces of foam. By rubbing the balloon, you charge the balloon with static electricity and once this happens, the balloon is surrounded by what’s called an electric field. Once again we can’t see an electric field, but we can certainly tell that it’s there.
You’re probably also familiar with static electricity. When you rub say a balloon on fabric, you can use it to attract little pieces of foam. By rubbing the balloon, you charge the balloon with static electricity and once this happens, the balloon is surrounded by what’s called an electric field. Once again we can’t see an electric field, but we can certainly tell that it’s there.
So what’s all this got to do with electromagnetic waves? Well electromagnetic waves are created by the vibrations of atoms and their electrons which creates a wave of magnetism (it’s called a magnetic-field wave) combined with a wave of static electricity (which is called an electric-field wave). Since there are two waves that combine into one, these kind of waves are called electromagnetic waves.
It may be hard to picture a magnetic field without a magnet, and it may be hard to picture an electric field without in this case a charged balloon, but in fact all electromagnetic waves are made of a travelling magnetic-field wave combined with a travelling electric-field wave.
 When electromagnetic waves strike something, they can reflect, or they can pass through the object, or they can be absorbed. When they are absorbed, the energy that they have actually causes the object to heat up.
When electromagnetic waves strike something, they can reflect, or they can pass through the object, or they can be absorbed. When they are absorbed, the energy that they have actually causes the object to heat up.
The sun produces mostly visible light (which we can feel as heat and see) and infrared light (which we can feel as heat but which we can’t see) and both contribute more or less equally to the heating of the Earth.
Hot things on Earth like radiant heaters give off mostly invisible infrared light and so it’s mostly the infrared light that warms us when we use them.
 This is a 10 Watt LED light globe. The Watt is the unit for power. 1 Watt = 1 Joule of energy per second, so a 10 W light globe is using 10 Joules of energy per second. This is a 2400 W radiant heater. It’s using 240 times the amount of electrical energy per second than the 10W light globe is using. It’s giving off a very small amount of visible light, mostly red and orange light, but it’s giving off a huge amount of infrared light. I can’t see it, but I can certainly feel it, because the invisible infrared waves are striking my skin and heating it up. The infrared waves are getting absorbed by the molecules that make up my skin and causing them to vibrate faster.
This is a 10 Watt LED light globe. The Watt is the unit for power. 1 Watt = 1 Joule of energy per second, so a 10 W light globe is using 10 Joules of energy per second. This is a 2400 W radiant heater. It’s using 240 times the amount of electrical energy per second than the 10W light globe is using. It’s giving off a very small amount of visible light, mostly red and orange light, but it’s giving off a huge amount of infrared light. I can’t see it, but I can certainly feel it, because the invisible infrared waves are striking my skin and heating it up. The infrared waves are getting absorbed by the molecules that make up my skin and causing them to vibrate faster.
The waves carry energy from the atoms of the heater to the atoms of my skin.
I might just re-emphasise this point. When any form of electromagnetic radiation light hits an atom, it can either reflect off it, pass through it or be absorbed by it. If it’s absorbed, the energy of the electromagnetic radiation will transfer to the atom.
 I can actually film the heater both with a normal visible-light camera and an infrared-detecting camera that can actually detect infrared light. As you can see, the heater is giving off a huge amount of invisible infrared light. When that light strikes a surface it warms the surface up. So, heat energy is being transferred from the heating elements to my skin on these infrared waves. This type of heat transfer is called radiant heat or heat radiation or quite simply radiation.
I can actually film the heater both with a normal visible-light camera and an infrared-detecting camera that can actually detect infrared light. As you can see, the heater is giving off a huge amount of invisible infrared light. When that light strikes a surface it warms the surface up. So, heat energy is being transferred from the heating elements to my skin on these infrared waves. This type of heat transfer is called radiant heat or heat radiation or quite simply radiation.
If we could see infrared light with our eyes, this 2400 W heater would be so absolutely blinding that it would basically be useless as a heater for, say, a bedroom. A small 10 W light globe is fairly bright, and a 50 W spotlight is very bright, so imagine how bright a 2400 W radiant heater would be if we could see infrared light.
If I switch the heater off, it starts to cool down to the point where it stops giving off visible light, but while it’s still quite hot, it’s still emitting a huge amount of infrared light and I can still feel it, even though I can’t see it with my eyes anymore.

 The heating element in this griller also emits a huge amount of radiant heat. It’s giving off a little bit of red light, but most of the energy it gives off is invisible infrared light. When I film in the dark, the visible red light is not all that bright. When I flick the switch over to the Infrared setting, the camera is overwhelmed by the amount of infrared light that hits it, and so it quickly has to auto-adjust.
The heating element in this griller also emits a huge amount of radiant heat. It’s giving off a little bit of red light, but most of the energy it gives off is invisible infrared light. When I film in the dark, the visible red light is not all that bright. When I flick the switch over to the Infrared setting, the camera is overwhelmed by the amount of infrared light that hits it, and so it quickly has to auto-adjust.
 In grilling, toasting, and barbecuing, the food is cooked mostly by the invisible radiant heat that the griller, toaster and hot barbecue coals produce. Sure the air heats up as well and this contributes a little bit of the heat transferring to the food, but it’s a very small factor compared to the radiant heat. This is especially true of a griller. Warm air rises, so it doesn’t really move towards the cheese being toasted here. The invisible but very intense infrared waves alone are doing the cooking here.
In grilling, toasting, and barbecuing, the food is cooked mostly by the invisible radiant heat that the griller, toaster and hot barbecue coals produce. Sure the air heats up as well and this contributes a little bit of the heat transferring to the food, but it’s a very small factor compared to the radiant heat. This is especially true of a griller. Warm air rises, so it doesn’t really move towards the cheese being toasted here. The invisible but very intense infrared waves alone are doing the cooking here.
So, all electromagnetic waves can transfer heat energy from one thing to another, but infrared is responsible for most of it, certainly on Earth anyway. (Any heat energy that is transferred by electromagnetic waves is called “radiant heat”, although when most people say radiant heat they’re often just talking about the invisible infrared light.)
Heat sources like radiant heaters, hot coals, grillers, and toasters produce 99.99% or so of their electromagnetic radiation in the form of invisible infrared light. When these waves are absorbed by something, they cause the object to heat up.
 The sun, our most important heat source, is an intensely bright heat source that warms the earth and everything on it with more or less equal amounts of visible light and infrared light, along with a little UV light as well.
The sun, our most important heat source, is an intensely bright heat source that warms the earth and everything on it with more or less equal amounts of visible light and infrared light, along with a little UV light as well.
In fact, of all the light that hits the surface of the Earth, a little over half is infrared light (53%), a little under half is visible light (44%), and about 3% is ultraviolet. As I said, all of it heats the Earth, but the visible light also allows us to see things.
 White surfaces reflect more light than dark surfaces, so they generally don’t get as hot as dark surfaces when the sun shines on them. These buildings on the famous Greek island of Santorini stay relatively cool in summer, despite the months of hot sunny weather that the island gets every year. The white paint reflects the sunlight and so, because the sunlight doesn’t get absorbed, the buildings stay cooler than if they had been painted darker colours. Darker things absorb light, so they get a little hotter in the sun.
White surfaces reflect more light than dark surfaces, so they generally don’t get as hot as dark surfaces when the sun shines on them. These buildings on the famous Greek island of Santorini stay relatively cool in summer, despite the months of hot sunny weather that the island gets every year. The white paint reflects the sunlight and so, because the sunlight doesn’t get absorbed, the buildings stay cooler than if they had been painted darker colours. Darker things absorb light, so they get a little hotter in the sun.
So, in summary, heat energy can transfer by
- conduction, when things are in direct contact, or by
- convection, that is by currents of warm air or water, or by
- radiation on electromagnetic waves that are absorbed by things which then heat up.
Now we are producing heat in our bodies continuously and yet we stay at 37°C give or take. How do we manage to achieve a more-or-less constant body temperature whether it’s really hot or really cold? Well, it’s a combination of clever technology and certain processes that happen within our bodies that we have little control over, and it’s these things that we’ll be looking at in our next episode. See you then.
CREDITS:
Thermal Conductivities taken from Engineering ToolBox (2003). Thermal Conductivity of common Materials and Gases. Available at: https://www.engineeringtoolbox.com/thermal-conductivity-d_429.html. Accessed 16/10/2018.



