The Shedding Light on Atoms series documents the development of our ideas about atoms while at the same time teaching students everything they need to know about modern chemistry. It explains not just what we know about atoms, but also how we know what we know about atoms.
We begin Episode 4: The Periodic Table, by comparing and contrasting metals with non-metals. We then describe how Dmitri Mendeleev, the scientist who devised the first Periodic Table, organised the elements not just into metals and non-metals but also into groups based on patterns in the way that they chemically react. Finally, we explain how Mendeleev was able to use the Periodic Table to predict the existence of, and many of the properties of, elements that hadn’t at the time been discovered!
The preview video below contains a 5-minute excerpt followed by a 2-minute trailer.
 The Episode 4 Question Sheet for Students:
The Episode 4 Question Sheet for Students:
![]() The PDF version.
The PDF version. ![]()
Google The Google Doc version. Google
![]() If you have ClickView, watch the whole episode here.
If you have ClickView, watch the whole episode here.
![]() If you have Learn360, watch the whole episode here.
If you have Learn360, watch the whole episode here.
![]() If you have Films on Demand, watch the whole episode here.
If you have Films on Demand, watch the whole episode here.
![]() If you have Classroom Video, watch the whole episode here.
If you have Classroom Video, watch the whole episode here.
![]() Most of our videos are also available on SAFARI Montage. Just log in and do a quick search.
Most of our videos are also available on SAFARI Montage. Just log in and do a quick search.
![]() Don’t have any of the above? Rent or buy the Shedding Light series and/or individual programs from our Vimeo page!!
Don’t have any of the above? Rent or buy the Shedding Light series and/or individual programs from our Vimeo page!!
 Here’s an A4 printable Periodic Table that that includes notes that will help students learn about isotopes, electron shell configurations, ions, and much more.
Here’s an A4 printable Periodic Table that that includes notes that will help students learn about isotopes, electron shell configurations, ions, and much more.
The pdf version.
The Word (docx) version.
The Transcript (which can be used as a textbook)
Contents:
Part A: Introduction: We look back briefly at the difference between elements and compounds and how Dalton came up with his atomic theory.
Part B: Classifying the Elements: Elements, at a simple level, can be classified into two main groups: metals and non-metals. Some of the main differences between the two are demonstrated.
Part C: Discovering with Electricity: Lavoisier’s 1780s list of elements contained only 24 elements. In 1800, Alessandro Volta invented the first battery. Electricity helped with the discovery of many new elements.
Part D: A Periodicity of Properties: By 1869, scientists had discovered 63 different elements and this was enough for them to start seeing lots of different patterns in the way that they behaved chemically. Dmitri Mendeleev produced a table—the first Periodic Table—which didn’t just classify the elements as metals and non-metals but organized them into groups based on how they chemically react with oxygen and hydrogen. We look at how he achieved this remarkable scientific breakthrough.
Part E : The Prediction of New Elements: Mendeleev’s organization of the elements into a Periodic Table was so good that he was able to predict the existence of elements that hadn’t been discovered and many of their chemical and physical properties.
Part A: Introduction
 Democritus, Black, Scheele, Priestly, Lavoisier, Bernoulli, Proust, Dalton, Avogadro. These are just some of the scientists whose ideas and experiments led to the discovery of atoms.
Democritus, Black, Scheele, Priestly, Lavoisier, Bernoulli, Proust, Dalton, Avogadro. These are just some of the scientists whose ideas and experiments led to the discovery of atoms.
By the late 1700s, it was clear that some substances like gold and oxygen could not be broken down into simpler substances. They were called elements. Other substances, like water and calcium carbonate, a major part of seashells, were made of two or more elements which had chemically joined together. These were called compounds.
It was discovered that the elements don’t join up in random amounts to form compounds but always join up in fixed proportions by weight.
For example, if 100 grams of zinc chemically combines with oxygen, it will always combine with exactly 24.47 grams of oxygen to produce 124.47 grams of zinc oxide, a white powder.
In 1802, John Dalton used all the data that he and others had collected to come up with his atomic theory.
He proposed that the element zinc is made up entirely of indestructible zinc atoms and that the element oxygen is made up entirely of indestructible oxygen atoms (though he didn’t know that the oxygen atoms in the air always come in pairs). Zinc oxide is formed when the zinc atoms bond to the oxygen atoms.
If the zinc and oxygen atoms bond in a 1:1 ratio, so that there are as many zinc atoms as there are oxygen atoms, then zinc atoms must be about 4 times heavier than oxygen atoms, since 100 grams is about 4 times heavier than 24.47 grams. (100/24.47 = 4.08 times heavier)
Atoms don’t break into pieces in chemical reactions, Dalton said, they simply rearrange themselves into new groups.
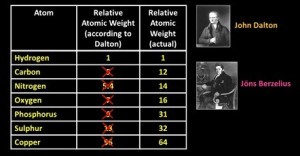 Dalton published a list of how heavy each different type of atom was compared to hydrogen atoms, since hydrogen atoms were the lightest of all atoms, but because initially no-one knew the exact ratio that the atoms joined up in, his list contained lots of errors. Over the next 20 years or so, more and more data was collected, especially by the Italian scientist Amadeo Avogadro and the Swedish scientist Jons Berzelius, and the list of the relative atomic weights that he published in 1826 was very accurate.
Dalton published a list of how heavy each different type of atom was compared to hydrogen atoms, since hydrogen atoms were the lightest of all atoms, but because initially no-one knew the exact ratio that the atoms joined up in, his list contained lots of errors. Over the next 20 years or so, more and more data was collected, especially by the Italian scientist Amadeo Avogadro and the Swedish scientist Jons Berzelius, and the list of the relative atomic weights that he published in 1826 was very accurate.
(http://chemed.chem.purdue.edu/genchem/history/berzelius.html)
 Berzelius also came up with our modern system of chemical notation, when he started using letters as atomic symbols to represent the different types of atoms, and chemical formulas to represent compounds, like water, H2O, carbon dioxide, CO2, one of the gases we exhale, and sea salt or sodium chloride, NaCl, just to name a few examples.
Berzelius also came up with our modern system of chemical notation, when he started using letters as atomic symbols to represent the different types of atoms, and chemical formulas to represent compounds, like water, H2O, carbon dioxide, CO2, one of the gases we exhale, and sea salt or sodium chloride, NaCl, just to name a few examples.
But scientists still had a lot to learn. How did atoms actually bond together and why did they bond together only in certain combinations? These questions remained unanswered for more than 100 years after Dalton first presented his evidence for the existence of atoms.
During the following 30 or 40 years or so, scientists began noticing that elements could be classified into groups based on how they behave chemically.
This work led to the development of the Periodic Table of the Elements in the 1860s, which not only listed the elements but also organized them in a logical way. The word “periodic” refers to the reoccurrence of something at a regular interval.
As it was being developed, it could be also used to predict the existence of certain elements which hadn’t been discovered yet. So in this episode we’ll be taking a look at the periodic table.
Part B: Classifying the Elements
Elements, at a really simple level, can be classified into two main groups: metals, like copper, and non-metals, like sulfur (or sulphur). (Sulfur and sulphur are both “correct” but sulfur has become the more common spelling.) Metals have certain characteristics in common as do non-metals.
 Firstly, all metals are said to be malleable, which means that they can be bent or hammered into different shapes. Some metals are fairly soft and bend easily (lead, Pb), while others are much harder (copper, Cu).
Firstly, all metals are said to be malleable, which means that they can be bent or hammered into different shapes. Some metals are fairly soft and bend easily (lead, Pb), while others are much harder (copper, Cu).
Bending this steel nail is fairly difficult, but when it’s hot, bending it becomes much easier, because heat makes metal more malleable.

 Most non-metals on the other hand, shaded pink on this Periodic Table are gases at normal temperatures. The solid ones, like carbon and sulfur (which is also spelled sulphur) for example are said to be brittle.
Most non-metals on the other hand, shaded pink on this Periodic Table are gases at normal temperatures. The solid ones, like carbon and sulfur (which is also spelled sulphur) for example are said to be brittle.
Brittle things crumble when too much pressure is applied.
Metals are also ductile, which means they can be drawn into wires, while non-metals are non-ductile.
 Metals are said to be lustrous, or shiny, while elements which are non-metals are fairly dull in comparison.
Metals are said to be lustrous, or shiny, while elements which are non-metals are fairly dull in comparison.
Metals are also good conductors of electricity, while non-metals are not. Here I’ve set up a circuit with a power pack, a light globe, an ammeter, which measures how much electric current is flowing, and a gap into which I can place different elements. 
Tin is a good conductor, electricity flows through it easily, as is nickel, iron, copper and zinc. However, sulfur, a non-metal, dos not conduct electricity and is said to be an insulator.
Carbon comes in different forms. One form, charcoal, does not conduct electricity, but graphite, the type of carbon found in pencils, does conduct electricity. Carbon is quite unusual for a non-metal in this respect.
 Finally, metals are good conductors of heat. As this iron rod is heated at one end, the heat passes through it, that is the heat conducts through it, and the blobs of wax holding up the nails each melt in turn. It took about five minutes for the last nail to fall. Non-metals are usually poor conductors of heat.
Finally, metals are good conductors of heat. As this iron rod is heated at one end, the heat passes through it, that is the heat conducts through it, and the blobs of wax holding up the nails each melt in turn. It took about five minutes for the last nail to fall. Non-metals are usually poor conductors of heat.
Scientists often use the word “property” to describe the characteristics of a substance. If you are asked to list the properties of metals, you would say that they are malleable, ductile, shiny or lustrous, and good conductors of electricity and heat. Non-metal elements are brittle (well the solid ones are), non-ductile, dull, and usually poor conductors of electricity and heat.
 On a periodic table, all the elements which are metals are placed on the left—as you can see, most elements are metals—and all the elements which are non-metals are placed over on the right. In between, are the so-called metalloids. Depending on the exact way the atoms are arranged within the sample, they can often be quite lustrous like metals, but they’re brittle like non-metals. However, there’s a lot more to the periodic table than just sorting out the metals, the non-metals and the metalloids.
On a periodic table, all the elements which are metals are placed on the left—as you can see, most elements are metals—and all the elements which are non-metals are placed over on the right. In between, are the so-called metalloids. Depending on the exact way the atoms are arranged within the sample, they can often be quite lustrous like metals, but they’re brittle like non-metals. However, there’s a lot more to the periodic table than just sorting out the metals, the non-metals and the metalloids.
Part C: Discovering with Electricity.

With the exception of O, N, H and S, all of the photos above are copyright Heinrich Pniok and are used with permission. Visit his website at http://pse-mendelejew.de/en/ to see his collection, arguably the finest on the internet. Many other photos used in the production of the Shedding Light on Atoms series were also taken by Heinrich Pniok.
Lavoisier’s original list of elements, published in the 1780s, contained only 23 elements, which is not really enough to notice any real patterns in the way that they all chemically react, although it was obvious that some were metals and some were non-metals.
In 1800, Italian scientist Alessandro Volta invented what was named the voltaic pile, the first electric battery. It was basically a stack of alternating copper discs, discs made of cardboard soaked in a salt solution and zinc discs.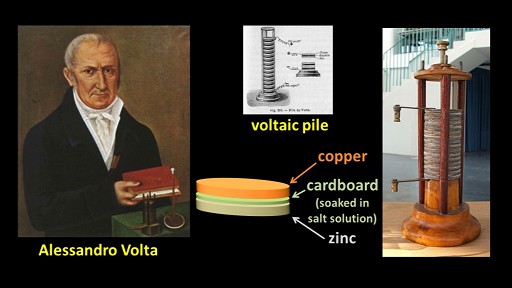
I can create a simplified voltaic pile with a piece of zinc and a piece of copper. If I dissolve some salt into the water, and then place the two metal plates into the salt solution, electricity is produced.
The voltaic pile was responsible for the discovery of many new elements.
This is copper oxide. In our last episode, we saw that if we mix carbon powder with copper oxide and then heat them in a test tube, a chemical reaction takes place and copper metal is formed. The chemical equation for the reaction is
copper oxide + carbon –> copper + carbon monoxide
CuO + C –> Cu + CO
I can also produce copper metal using electricity.
I’ll first pour some sulfuric acid into a beaker and then tip in some copper oxide. If I then slowly heat the acid, all the copper oxide eventually dissolves. It takes about five minutes or so. Though copper oxide is black, it turns blue when it dissolves in the acid.

 I can now pass electricity through the solution using two carbon electrodes.
I can now pass electricity through the solution using two carbon electrodes.
The carbon is in the form of graphite, which as I said earlier, conducts electricity. I’m using a power pack instead of a battery. Copper metal immediately begins to form on the electrode connected to the negative terminal of the battery. About ten minutes later, quite a lot of copper has formed.
The process of using electricity in this way to drive chemical reactions is called electrolysis.
English scientist Humphry Davy used electrolysis to discover many new elements (sodium, potassium, calcium, magnesium, boron, barium and strontium) in 1807 and 1808.
He produced sodium metal for example by passing electricity through molten sodium chloride: everyday table salt. It doesn’t work if you just dissolve the table salt in water or in acid because the water ends up chemically reacting instead of the sodium chloride.
Aluminium (or aluminum if you prefer), was discovered by electrolysis in 1825 by Hans Christian Ørsted. Until the 1880s, it was more expensive than gold, but then American Charles Martin Hall and Frenchman Paul Héroult discovered a way of producing it relatively cheaply and it’s still made in much the same way today.


 Aluminium comes from a mineral called bauxite. Bauxite contains significant amounts of a substance called alumina, which is actually aluminium oxide (Al2O3).
Aluminium comes from a mineral called bauxite. Bauxite contains significant amounts of a substance called alumina, which is actually aluminium oxide (Al2O3).
The alumina is extracted from the bauxite in huge processing plants.
Now to extract copper from copper oxide I dissolved the copper oxide in acid. Aluminium though, can’t be extracted by dissolving the alumina in acid. The alumina instead is dissolved in molten cryolite (Na3AlF6), a mineral with sodium, aluminium and fluorine in it. This takes place in huge so-called “cells” or “pots” at temperatures of about 1000°C (1830°F). Electricity is made to pass through the molten alumina and cryolite mixture through huge carbon blocks, similar to what I showed you with the dissolved copper oxide.
The cell is so hot that the aluminium that forms on the bottom carbon block is in a molten state and so stays at the bottom of the cell, with the alumina-cryolite mixture floating above it. It’s then siphoned out and shaped into so-called ingots of various shapes and sizes.
 Aluminium is fairly strong and yet it’s fairly lightweight compared to most metals. It’s used to make aircraft bodies, high-voltage transmission lines, drink cans, and a whole lot of other things. In fact, it’s the second-most widely used metal in the world, iron being the most widely used.
Aluminium is fairly strong and yet it’s fairly lightweight compared to most metals. It’s used to make aircraft bodies, high-voltage transmission lines, drink cans, and a whole lot of other things. In fact, it’s the second-most widely used metal in the world, iron being the most widely used.
Part D: A Periodicity of Properties
By 1869, scientists had, using electrolysis and other chemical techniques, discovered 63 different elements and this was enough for them to start seeing lots of different patterns or trends in the way that they behaved chemically.
Now you could just list the elements in order from lightest to heaviest, but then any trends would not be clear. Remember, no-one knew how heavy atoms were, they only knew how heavy they were compared to each other. Hydrogen, being the lightest of all atoms was given a weight of 1.
 In 1869, Russian scientist Dmitri Ivanovich Mendeleev arranged the then known elements not only in order of increasing atomic weight, but also in a table which made the trends really obvious. Let’s have a look at some of these trends.
In 1869, Russian scientist Dmitri Ivanovich Mendeleev arranged the then known elements not only in order of increasing atomic weight, but also in a table which made the trends really obvious. Let’s have a look at some of these trends.
 This is lithium, a type of metal. It has to be stored under oil because it reacts very quickly with oxygen and with water. It’s soft enough to be cut with a knife. You can see the pure, lustrous lithium metal once I’ve cut through it, but the luster doesn’t last long because it reacts so quickly.
This is lithium, a type of metal. It has to be stored under oil because it reacts very quickly with oxygen and with water. It’s soft enough to be cut with a knife. You can see the pure, lustrous lithium metal once I’ve cut through it, but the luster doesn’t last long because it reacts so quickly.
The equation for lithium’s reaction with oxygen is
lithium + oxygen –> lithium oxide
Li + O2 –> Li2O
 We can see that for every pair of oxygen atoms, 2 Li2Os are produced, which means that we need a total of four lithium atoms to react with every pair of oxygen atoms. The balanced equation is therefore
We can see that for every pair of oxygen atoms, 2 Li2Os are produced, which means that we need a total of four lithium atoms to react with every pair of oxygen atoms. The balanced equation is therefore
4 Li + O2 –> 2 Li2O
Lithium reacts readily with water. The equation for the reaction is
lithium + water ? hydrogen (that’s what’s in the bubbles being produced) + lithium hydroxide (which remains dissolved in the water so we can’t see it). In symbols,
2 Li + 2 H2O –> H2 + 2 LiOH
 This is sodium. Sodium is also, like lithium, a soft, silvery metal which is very reactive, more so in fact than lithium.
This is sodium. Sodium is also, like lithium, a soft, silvery metal which is very reactive, more so in fact than lithium.
It quickly corrodes in the air to produce the light grey sodium oxide layer that you can see on this sample.
The equation for sodium’s chemical reaction with oxygen is
sodium + oxygen –> sodium oxide
4 Na + O2 –> 2 Na2O

 It also reacts with water in the same way as lithium does, but even more vigorously. The equation is
It also reacts with water in the same way as lithium does, but even more vigorously. The equation is
sodium + water –> hydrogen + sodium hydroxide.
2 Na + 2 H2O –> H2 + 2 NaOH.
Quite often, so much heat is generated in the reaction that the hydrogen ignites.
In fact, a largish chunk of sodium… will explode.
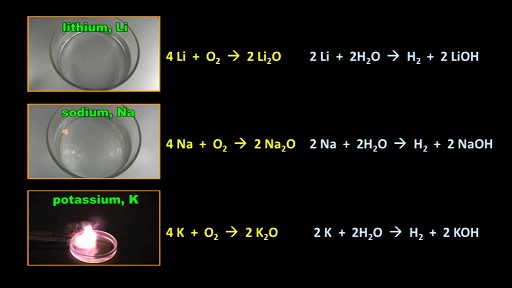 Notice that the chemical equations for the reactions of the two metals with oxygen are the same, and so are the equations for their reactions with water.
Notice that the chemical equations for the reactions of the two metals with oxygen are the same, and so are the equations for their reactions with water.
Potassium is another metal which is very, very reactive. The chemical equations for its reactions with oxygen and with water are the same again.
Clearly these metals belong together in a group so Mendeleev organized his periodic table to place these metals in a group. Here they are, here: Lithium, Sodium and Potassium.
So how did he come up with the whole table? Let’s have a look.
 He first placed hydrogen at the top of what he called Group 1. He knew that when hydrogen reacts with oxygen it produces water, H2O. The ratio of hydrogen atoms to oxygen atoms in H2O is 2:1. Why is this important? Well, the reaction of each element with oxygen was one of the keys to organizing the elements into a periodic table, as we’re about to see.
He first placed hydrogen at the top of what he called Group 1. He knew that when hydrogen reacts with oxygen it produces water, H2O. The ratio of hydrogen atoms to oxygen atoms in H2O is 2:1. Why is this important? Well, the reaction of each element with oxygen was one of the keys to organizing the elements into a periodic table, as we’re about to see.
The next heaviest element is Helium, but Helium hadn’t been discovered. The next heaviest known element was Lithium, whose atoms are 7 times heavier than hydrogen atoms. Since Lithium produces Li2O when it reacts with oxygen, a 2:1 ratio of Lithium atoms to oxygen atoms, Mendeleev decided to put Lithium in the same group as hydrogen. He then arranged Beryllium, Boron, Carbon, Nitrogen, Oxygen and Fluorine in a horizontal row in order of increasing atomic weight. He called each horizontal row a Period, and we still use this word today. The next heaviest atoms are those of Neon, but Neon, like Helium, had not yet been discovered. The next heaviest atoms that had been discovered were those of sodium, which as we’ve seen is similar chemically speaking to lithium, so sodium was placed into a new Period, Period 3, under lithium.
When sodium reacts with oxygen, the product is Na2O, once again a 2:1 ratio of sodium atoms to oxygen atoms.
Into this third Period, he then placed magnesium, aluminium, silicon, phosphorus, sulfur and chlorine, again in order of increasing atomic weight.
In 1871, Argon hadn’t been discovered, so potassium came next. Since potassium is similar to sodium, it was placed in a new Period under sodium. When it reacts with oxygen, it produces K2O with the same now-familiar 2:1 ratio. Calcium was next, but let’s stop there for now.
It’s clear that the Group 1 elements had similarities, but what about the elements in the other columns, which were called Group 2, Group 3 and so on up to Group 7.
 When the atoms of the elements in Group 2 bonded with oxygen atoms to form oxides (an oxide is any compound made of oxygen and another element, like magnesium oxide), they would always do so in a 1:1 ratio.
When the atoms of the elements in Group 2 bonded with oxygen atoms to form oxides (an oxide is any compound made of oxygen and another element, like magnesium oxide), they would always do so in a 1:1 ratio.
The atoms of the Group 3 elements always bonded in a 2:3 ratio with oxygen atoms, the atoms of the Group 4 elements in a 1:2 ratio, and the atoms of the Group 5 elements in a 2:5 ratio.
Unfortunately this particular pattern didn’t hold true for the Group 6 and 7 elements but there were other similarities that we’ll look at soon.
Now there weren’t just similarities within each group, there was also a regular pattern across the periods. Can you see the mathematical pattern?
 If, in Group 1, we divide the number of oxygen atoms in each compound by the number of the other atoms that oxygen joins with, for example with H2O, 1 oxygen atom divided by 2 hydrogen atoms, we get one half. This of course applies to all the Group 1 elements. In the next Group, if we divide the number of oxygen atoms in each oxide by the number of the atoms of the Group 2 elements, 1 divided by 1, we get 1. We then get 1½, 2, 2½, 3, and 3½.
If, in Group 1, we divide the number of oxygen atoms in each compound by the number of the other atoms that oxygen joins with, for example with H2O, 1 oxygen atom divided by 2 hydrogen atoms, we get one half. This of course applies to all the Group 1 elements. In the next Group, if we divide the number of oxygen atoms in each oxide by the number of the atoms of the Group 2 elements, 1 divided by 1, we get 1. We then get 1½, 2, 2½, 3, and 3½.
Mendeleev couldn’t explain why this pattern occurred. All he could say was that “The elements, if arranged according to their atomic weight, exhibit an apparent periodicity of properties.” In other words, there were patterns in the way they chemically react.
By the way, the oxides we’ve shown for carbon, nitrogen, phosphorus and chlorine are not the only oxides that form between these atoms and oxygen but they’re the ones with the highest ratios.
 Now Mendeleev also noticed patterns in the way that the Group 4 to Group 7 elements produced compounds with hydrogen. This was another key to organizing the elements successfully.
Now Mendeleev also noticed patterns in the way that the Group 4 to Group 7 elements produced compounds with hydrogen. This was another key to organizing the elements successfully.
A single atom of carbon can only ever join with 4 atoms of hydrogen, no more and no less, to produce CH4, nitrogen atoms only ever join with 3 H atoms to produce NH3, oxygen atoms only ever join with two H atoms to produce H2O, and fluorine atoms only ever join with one H atom to produce HF. The ratios of atoms go from 4:1, to 3:1 to 2:1, to 1:1.
This exact same pattern is repeated in the next period: Silicon atoms react with 4 H atoms to produce SiH4; phosphorus atoms with 3 H atoms to produce PH3; sulfur atoms with 2 H atoms to produce H2S; and chlorine atoms with 1 H atom to produce HCl.
Once again, he didn’t know why the atoms joined together in these ratios and why the patterns, or the periodicity existed, but he was clearly onto something big.
Part E : The Prediction of New Elements.
Recognizing patterns is a really important part of Science. Patterns can help you to gain a deeper understanding of nature and can help you to make predictions. For example, the progression of the seasons: summer, autumn (or fall), winter and spring is a pattern that people have been familiar with for thousands of years. Knowing about the regularity of the seasons allows us to work out the best times to plants crops and when to plan our holidays.
Classification is also important. By finding similarities in things, you can organize them into groups and be confident that what you discover about one of the things might also apply to the other things as well.
Now Mendeleev didn’t just find patterns in the order of the elements, he was also able to predict the existence of elements that hadn’t been discovered yet, as well as some of their chemical and physical properties. A property of a substance is a feature or characteristic like reactivity, melting point, density, strength, and so on.
Let’s have a look at two of the predictions he made.
Here we can see the first 4 periods of Mendeleev’s table. We’ll get rid of Period 4 just to save space, and analyze Period 5. We’ll also show the oxides which form when all these elements react with oxygen.
Let’s begin with zinc. When it reacts with oxygen, it produces zinc oxide, ZnO. So, given this information, in which Group does it belong? Naturally, Mendeleev placed zinc into Group 2 of his table, along with all the other elements whose atoms react in a 1:1 ratio with oxygen atoms.
After zinc, the next heaviest atoms that were known at the time were those of arsenic. with an atomic weight of 75. The highest oxide of arsenic is As2O5. So in which Group does arsenic belong? Mendeleev of course placed it into Group 5 underneath nitrogen and phosphorus. Selenium came next, and since it forms SeO3 when it reacts with oxygen, you’ve probably guessed what I’m about to say, it was placed into Group 6.
 The pattern worked well, so Mendeleev predicted that the gaps in the table, here and here, would eventually be filled by two elements which hadn’t been discovered. They would have atomic weights of about 68 and 72. He gave the two elements the temporary names of ekaaluminium and ekasilicon. The word eka means one in the ancient Sanskrit language. The two new elements would occupy one space below aluminium and silicon on his table (and they would produce oxides with the formulas Ea2O3 and EsO2).
The pattern worked well, so Mendeleev predicted that the gaps in the table, here and here, would eventually be filled by two elements which hadn’t been discovered. They would have atomic weights of about 68 and 72. He gave the two elements the temporary names of ekaaluminium and ekasilicon. The word eka means one in the ancient Sanskrit language. The two new elements would occupy one space below aluminium and silicon on his table (and they would produce oxides with the formulas Ea2O3 and EsO2).
Mendeleev was able to predict what these elements would be like based on, once again, looking at patterns in the known elements.
 Let’s look at density. The density of a substance equals its mass divided by its volume.
Let’s look at density. The density of a substance equals its mass divided by its volume.
For example, the mass of this aluminium cube is 42.2 grams. It’s volume is 2.5cm by 2.5 cm by 2.5 cm which equals 15.625cm3. Its density is therefore 42.2 grams over 15.625 grams which equals 2.7 grams per cubic centimetre.
Zinc has a much higher density of 7.1 g/cm3.

 This graph shows the densities of all the elements. You can see that there’s a fairly regular pattern of peaks and troughs.
This graph shows the densities of all the elements. You can see that there’s a fairly regular pattern of peaks and troughs.
In 1871 though, only these elements were known. The density of zinc, shown here, is 7.1g/cm3, while the density of arsenic is 5.7 g/cm3. Mendeleev predicted that the densities of the two undiscovered elements would lie somewhere in this region. The densities wouldn’t just jump up to here somewhere for example or drop down to here.
Based on patterns in, not just density, but other properties as well, he predicted that ekaaluminium would have an atomic weight of 68, a density of 6.0 grams/cm3, a low melting point and a reaction with oxygen that would produce Ea2O3 just as aluminium above it on his Periodic Table produces Al2O3 when it reacts with oxygen. He also predicted that ekaaluminium oxide’s (Ea2O3’s) density would be 5.5 grams/cm3.
 In 1875, French scientist Paul-Emile Lecoq discovered a new element which he named Gallium. Gallium had an atomic weight of 70, so the prediction of a new element in Period 5 was verified. Great news for Mendeleev! Lecoq wrote a letter to Mendeleev telling him of the discovery, but that, unfortunately, Gallium’s density was only 4.7 grams/cm3, well below the prediction, and a blow to Mendeleev’s theories. Bad news for Mendeleev.
In 1875, French scientist Paul-Emile Lecoq discovered a new element which he named Gallium. Gallium had an atomic weight of 70, so the prediction of a new element in Period 5 was verified. Great news for Mendeleev! Lecoq wrote a letter to Mendeleev telling him of the discovery, but that, unfortunately, Gallium’s density was only 4.7 grams/cm3, well below the prediction, and a blow to Mendeleev’s theories. Bad news for Mendeleev.
However, Mendeleev, wrote back to Lecoq saying that Lecoq had probably made a mistake (or a LeCoq up, as the English might say) and requested that he measure Gallium’s density again.
The Russian had never even seen Gallium, it had only just been discovered in France 2000km away, but he just knew that a density of 4.7 couldn’t be right, because it didn’t fit the pattern.
Lecoq, who had originally isolated only tiny amounts of Gallium, then produced more of it and did measure its density again, calculating a value of 5.9 g/cm3, which is a very close fit. Great news for Mendeleev again!
Gallium also had a low melting point of 30°C, it reacted with oxygen to produce Ga2O3, and Ga2O3’s density was 5.9 g/cm3.
The incredible accuracy of Mendeleev’s predictions made him famous in the scientific community and scientists now knew that the Periodic Table provided a really great way of organizing and studying elements.
 Mendeleev also predicted that the next unknown element, Ekasilicon, would have an atomic weight of 72, a density of 5.5 g/cm3, a high melting point, would react with oxygen to produce EsO2 and that EsO2’s density would be 4.7 g/cm3.
Mendeleev also predicted that the next unknown element, Ekasilicon, would have an atomic weight of 72, a density of 5.5 g/cm3, a high melting point, would react with oxygen to produce EsO2 and that EsO2’s density would be 4.7 g/cm3.
Fifteen years later, in 1886, German scientist Clemens Alexander Winkler discovered a new element which he called Germanium. Germanium had similar properties to what Mendeleev had predicted. It had an atomic weight of 73, a density of 5.35 g/cm3, a high melting point of 947°C, and an oxide formula of GeO2. GeO2’s density was 4.7 g/cm3.
Mendeleev also predicted the existence and many of the properties of many other elements and he got a lot of them right. (scandium, technetium, rhenium, francium, radium, protactinium and hafnium).
Though his Periodic Table had a few errors in it, and it couldn’t fully account for some elements, like these ones that he placed into Group 8 here, because he didn’t know where else to put them, it was never-the-less a brilliant achievement in science and provided a fantastic springboard for new discoveries.
As more discoveries were made, the periodic table was modified into the form that we have today. This form gives a much better account of the patterns that can be seen in the elements.
The mystery though, of why the elements behave chemically in these patterns remained until the late 1800s and early 1900s when scientists discovered that atoms themselves were made up of even smaller particles called protons, neutrons, and electrons, so it’s the discovery of these particles that we’ll be looking at in our next episode. See you then.

1. Zinc is made entirely of ____________ atoms and oxygen is made entirely of ______________ atoms. Zinc oxide (ZnO) is made of …
2. In the 1810s and 1820s, carbon was found to have a relative atomic weight of 12 and nitrogen a relative atomic weight of 14. What does “relative atomic weight” mean, and what do these figures tell you about carbon atoms and nitrogen atoms?
3. What happens to atoms in a chemical reaction?
4. Fill in the table with some of the properties of metals and non-metals. (A “property” of a substance is a characteristic that can be used to describe it.)

5. What is a metalloid? Give two examples.
6. The process of using electricity to drive chemical reactions is called _______________________.
7. Briefly describe how aluminium is produced in industry.
8. Elements within Groups usually chemically react in similar ways. Fill in the table below. (And balance the equations.)

9. Mendeleev predicted that ekaaluminium, Ea, and ekasilicon, Es, (the temporary names he gave to the undiscovered elements between zinc and arsenic) would react with oxygen to produce Ea2O3 and EsO2 respectively. On what did he base these predictions?
10. On reacting with hydrogen atoms, a single carbon atom will form CH4, a single nitrogen atom will form NH3, a single oxygen atom will form H2O, and a single fluorine atom will form HF. (Note the 4:1, 3:1, 2:1 and 1:1 ratios) Fill in the table below. (The first row has been done for you, the answers to the second row are in the video, but you have to “do a Mendeleev” and “guess” the third row.)

11. Graph the data in the table and then estimate the density of titanium (Ti). Mark in titanium’s data point.


12. Trends in the Periodic Table are apparent both across each Period and down each Group. Using two lines (one for boiling point and one for melting point), graph the data in the table on the right onto the graph below. Use a colour code. Use the graph to estimate the melting point and boiling point of bromine. Mark in bromine’s data points.


Credits:
Thanks to Heinrich Pniok at http://pse-mendelejew.de/en/ for his kind permission in allowing us to use his copyrighted images of the elements. Visit his website to view what is arguably the internet’s best collection of photos of elements. See also http://commons.wikimedia.org/wiki/User:Alchemist-hp?uselang=fr.
Polonium and Astatine images by images-of-elements.com (http://images-of-elements.com/astatine.php and http://images-of-elements.com/polonium.php) licensed under a Creative Commons Attribution 3.0 Unported License.
816- Ivigtut – cryolite.jpg (http://en.wikipedia.org/wiki/File:816-_Ivigtut_-_cryolite.jpg) by Didier Descouens is licensed under CC BY-SA 3.0.
Pila di Volta 01.jpg (http://commons.wikimedia.org/wiki/File:Pila_di_Volta_01.jpg) at the Tempio Voltiano, Como, Italy by Luigi Chiesa is licensed under CC BY-SA 3.0.
Potassium water 20.theora.ogv by Ozone aurora / Philip Evans is licensed under CC BY-SA 3.0.
Thanks to Phillip Island Nature Parks (http://www.penguins.org.au/), Phillip Island, Victoria.
Thanks to Alcoa for the footage of the production of Aluminium.
Written and presented by Spiro Liacos



