Nuclear radiation can be incredibly dangerous, but it can also be incredibly useful to us. The Shedding Light on Nuclear Radiation series teaches students what nuclear radiation is and how humans have harnessed its awesome power.
In Shedding Light on Nuclear Radiation Episode 3: Beta-Minus and Gamma Radiation, we look at what beta-minus and gamma radiation are and at how they are put to good use in medicine and in industry.
 The Episode 3 Question Sheet for Students:
The Episode 3 Question Sheet for Students:
![]() The PDF version.
The PDF version. ![]()
Google The Google Doc version. Google
Get the answers.
![]() If you have ClickView, watch the whole episode here.
If you have ClickView, watch the whole episode here.
![]() If you have Learn360, watch the whole episode here.
If you have Learn360, watch the whole episode here.
![]() If you have Classroom Video on Demand, watch the whole episode here.
If you have Classroom Video on Demand, watch the whole episode here.
![]() If you have Access Video on Demand, watch the whole episode here.
If you have Access Video on Demand, watch the whole episode here.
![]() You can also watch the episode on Safari Montage. (We can’t provide a link because each school has a different URL.)
You can also watch the episode on Safari Montage. (We can’t provide a link because each school has a different URL.)
![]() Don’t have any of the above? Rent or buy the Shedding Light on Nuclear Radiation series (or individual programs) on Vimeo!!
Don’t have any of the above? Rent or buy the Shedding Light on Nuclear Radiation series (or individual programs) on Vimeo!!
Contents:
Part A: Introduction
Part B: Beta-Minus Decay
Part C: Gamma Radiation
Part D: Comparing Alpha, Beta, and Gamma Radiation
Part E: Putting Beta-Minus and Gamma Radiation to Work
Transcript (more or less) (which can be used as a text book)
 Part A: Introduction
Part A: Introduction
Hi everyone. In this series we’re taking a look at nuclear radiation. Nuclear radiation is made of particles or electromagnetic waves that are emitted from the nuclei of certain types of atoms.
 In this episode of the series, we’re going to examine what beta and gamma radiation are, and we’ll look at some examples of how beta and gamma emitters are used in medicine and in industry.
In this episode of the series, we’re going to examine what beta and gamma radiation are, and we’ll look at some examples of how beta and gamma emitters are used in medicine and in industry.
Let’s begin this lesson by taking a look at beta decay. Now, the word beta is often pronounced as “bayta” (or beyta depending on your accent) but I’m going to be saying “beeta”. Let’s go.
Part B: Beta-Minus Decay
There are actually two types of beta decay: beta-minus and beta-plus decay. In this lesson we’re only going to look at beta-minus decay.

 We know that atoms are made of protons, neutrons, and electrons.
We know that atoms are made of protons, neutrons, and electrons.
However, if an atom has too many neutrons in its nucleus, one of the neutrons can literally change into a proton and an electron, and the electron flies off at really high speeds. This electron is called a beta-minus particle. Let’s look at an example.
We saw in Episode 1 that there are three naturally occurring isotopes of carbon. Carbon-14 which accounts for only about 1 in every trillion carbon atoms on earth is a beta-minus emitter.
 Let me show a close-up of a carbon-14 atom’s nucleus with protons in blue and neutrons in red, duplicate it, and examine what happens when it decays. In beta-minus decay, one of the neutrons turns into a proton and an electron. The electron is emitted from the nucleus at a speed of about 0.9 the speed of light (about 270,000 km/s) which is extremely fast of course, and it is called a beta-minus particle. A beta-minus particle is literally identical to an ordinary electron.
Let me show a close-up of a carbon-14 atom’s nucleus with protons in blue and neutrons in red, duplicate it, and examine what happens when it decays. In beta-minus decay, one of the neutrons turns into a proton and an electron. The electron is emitted from the nucleus at a speed of about 0.9 the speed of light (about 270,000 km/s) which is extremely fast of course, and it is called a beta-minus particle. A beta-minus particle is literally identical to an ordinary electron.
 However, since as soon as it’s created in the decay process, it is emitted at such high speeds, it pushes the electrons of other atoms away (remember, electrons repel each other because they’re all negatively charged) and this causes those atoms to become ions.
However, since as soon as it’s created in the decay process, it is emitted at such high speeds, it pushes the electrons of other atoms away (remember, electrons repel each other because they’re all negatively charged) and this causes those atoms to become ions.
So a beta-minus particle (or beta-minus radiation; both expressions are fairly common and mean the same thing), is a form of ionizing radiation, just like alpha radiation is. Ionizing radiation, as we saw in our previous episode, can kill living cells by upsetting the chemistry that’s going on in our cells.
Let’s watch the beta-minus decay process again and then I’ll show you how to write the nuclear equation for this decay. One of the neutrons changes into a proton and an electron, and the electron (which we refer to as a beta-minus particle) flies off at an enormous speed. We’ve seen that the carbon-14 nucleus can be written as 14
6C.
 Beta-minus particles are given the Greek symbol
Beta-minus particles are given the Greek symbol ![]() – (beta minus, the minus sign is a superscript) or e–, e for electron. When I handwrite the beta symbol, I use a curvy style like my Greek-school teachers taught me back in the day. However, you can also write it starting from below the line and then moving up and around. In atomic notation beta-minus particles are written as 0
– (beta minus, the minus sign is a superscript) or e–, e for electron. When I handwrite the beta symbol, I use a curvy style like my Greek-school teachers taught me back in the day. However, you can also write it starting from below the line and then moving up and around. In atomic notation beta-minus particles are written as 0
-1![]() or 0
or 0
-1e. The “-1” refers to the fact that they have a negative charge. Up until now, I’ve said that the 6 of, say, 14
6C
refers to the number of protons, which is true, but each proton has a charge of 1+, so the atomic number of 6 also refers to the number of positive charges in the nucleus. The -1 therefore in 0
-1![]() refers to the fact that the particle has a charge of minus 1. The “0” means that the combined number of protons and neutrons is zero. So, what particle has a charge of -1 and is not made up of protons and neutrons. Well, an electron!
refers to the fact that the particle has a charge of minus 1. The “0” means that the combined number of protons and neutrons is zero. So, what particle has a charge of -1 and is not made up of protons and neutrons. Well, an electron!
So, let’s write the nuclear equation. There are 6 protons and 8 neutrons in the nucleus of a carbon-14 atom, which here is the parent nucleus. A beta-minus particle is emitted which we can write in and we can then determine what the daughter nucleus is. One of the neutrons has turned into a proton so the nucleus now has one more proton than it had originally. Instead of 6, it’s got 7. And what type of an atom has 7 protons? If we look at the periodic table, we can see that it’s a nitrogen atom. We can now write in an N. Now, the combined number of protons and neutrons is still the same. So, we can write 14 here. The carbon-14 atom has turned into a nitrogen-14 atom and a beta minus particle has been emitted.
14
6C
—> 14
7N
+ 0
-1![]()
That’s the nuclear equation for the beta-minus decay of carbon-14. Notice that 6 = 7 + -1 and that 14 = 14 + 0. The mathematics is pretty simple.
We saw in our last episode that alpha particles are stopped by a few centimetres of air and they can’t penetrate our skin. Beta-minus particles are a little more penetrating, but clothes or a few layers of aluminium foil can stop them. Gamma rays can go straight through our bodies no problem. What are gamma rays? Let’s take a look.
Part C: Gamma Radiation
Gamma radiation is the third common form of radiation. Gamma rays are not particles but electromagnetic waves; high energy, highly penetrating, electromagnetic waves.
Electromagnetic waves include radio waves, microwaves, infrared waves ordinary visible light (which we can see with our eyes), ultraviolet light, X-rays and gamma rays.

 Now most of us are familiar with X-rays. X-rays can penetrate our skin and our muscles but not our bones, so we can produce X-ray photos using X-rays. Gamma rays have an even higher penetrating ability than X-rays and can go straight through bones.
Now most of us are familiar with X-rays. X-rays can penetrate our skin and our muscles but not our bones, so we can produce X-ray photos using X-rays. Gamma rays have an even higher penetrating ability than X-rays and can go straight through bones.
Gamma rays are not usually emitted on their own; they usually get emitted at the same time as either an alpha or a beta-minus particle.
 For example, cobalt-60 is a beta-minus and gamma emitter. Let me duplicate it. It emits a beta-minus particle and then within about a trillionth of a second, it also emits a gamma ray. The symbol for a gamma ray is the Greek letter gamma, which is the third letter of the Greek alphabet. Alpha, beta and gamma by the way are the first three letters of the Greek alphabet. When you handwrite the letter gamma, it looks something like this.
For example, cobalt-60 is a beta-minus and gamma emitter. Let me duplicate it. It emits a beta-minus particle and then within about a trillionth of a second, it also emits a gamma ray. The symbol for a gamma ray is the Greek letter gamma, which is the third letter of the Greek alphabet. Alpha, beta and gamma by the way are the first three letters of the Greek alphabet. When you handwrite the letter gamma, it looks something like this.
Let’s write a nuclear equation to express what happens. The atomic notation for cobalt-60 is 60
27Co
, and for the beta-minus particle is 0
-1![]()
, as we’ve seen. Gamma rays are written as 0
0![]()
in atomic notation, so we can add that to the equation. They have no charge and no mass since they’re a form of electromagnetic radiation. So, let’s finish with the daughter nucleus. In the beta-minus decay, one of the neutrons turns into a proton and so the nucleus ends up with 28 protons so we can write that in. If we consult a Periodic Table, we can see that nickel atoms have 28 protons, so, back to the equation, we can write in Ni. The mass number stays the same, 60, so it is in fact a nickel-60 atom.
60
27Co
—> 60
28Ni
+ 0
-1![]()
+ 0
0![]()
So, we now have a complete equation for the beta-minus and gamma decay of cobalt-60. Notice that 27 = 28 + -1 + 0 and that 60 = 60 + 0 + 0.
 Now gamma rays, like alpha and beta-minus particles, can also knock electrons off atoms. In other words, gamma radiation is a type of ionising radiation. Gamma rays are less ionizing than alpha and beta-minus particles, but they have a far greater penetrating ability, which makes them very dangerous to living organisms.
Now gamma rays, like alpha and beta-minus particles, can also knock electrons off atoms. In other words, gamma radiation is a type of ionising radiation. Gamma rays are less ionizing than alpha and beta-minus particles, but they have a far greater penetrating ability, which makes them very dangerous to living organisms.
Now before we look at some examples of how beta-minus and gamma rays can be useful to us, let’s compare all three forms of radiation that we’ve looked at so far.
Part D: Comparing Alpha, Beta, and Gamma Radiation.
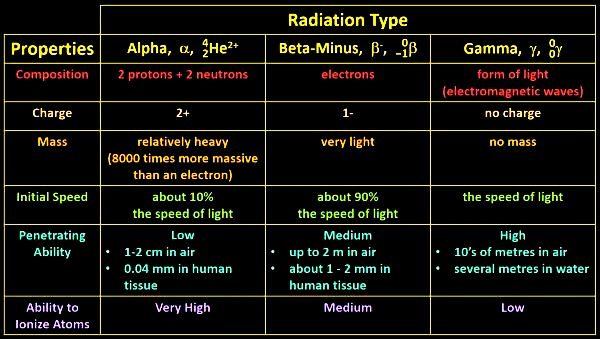 So, let’s compare some of the properties of alpha, beta-minus, and gamma radiation side by side. Alpha particles are made of 2 protons and 2 neutrons which means that they’re the same as the nucleus of a helium atom. Beta-minus particles are electrons, and gamma rays are a form of light, that is they’re electromagnetic waves. Alpha particles have a double positive charge, beta-minus particles a single negative charge, and gamma rays have no charge. Alpha particles are relatively heavy, being some 8000 times heavier than beta-minus particles, which relatively speaking are very light. Gamma rays have no mass at all. Alpha particles are emitted at about 10% of the speed of light, beta particles at about 90% of the speed of light, and gamma rays at the speed of light, because they are form of light.
So, let’s compare some of the properties of alpha, beta-minus, and gamma radiation side by side. Alpha particles are made of 2 protons and 2 neutrons which means that they’re the same as the nucleus of a helium atom. Beta-minus particles are electrons, and gamma rays are a form of light, that is they’re electromagnetic waves. Alpha particles have a double positive charge, beta-minus particles a single negative charge, and gamma rays have no charge. Alpha particles are relatively heavy, being some 8000 times heavier than beta-minus particles, which relatively speaking are very light. Gamma rays have no mass at all. Alpha particles are emitted at about 10% of the speed of light, beta particles at about 90% of the speed of light, and gamma rays at the speed of light, because they are form of light.
Alpha particles have a low penetrating ability. They can travel about 1-2 cm in air or about 0.04 mm in human tissue before they slow down to the point where they can no longer cause ionisations.


 This Geiger counter is detecting alpha particles being emitted by the tiny speck of Americium-241 that is inside the steel housing. If I lift the detector even a little bit, the count rate drops right down. As I said, a few centimetres of air is enough to stop them.
This Geiger counter is detecting alpha particles being emitted by the tiny speck of Americium-241 that is inside the steel housing. If I lift the detector even a little bit, the count rate drops right down. As I said, a few centimetres of air is enough to stop them.
Two sheets of paper, with a thickness of about a quarter of a millimetre, also stops nearly all of the alpha particles. When I remove the paper, the counter goes into overdrive.
So, just repeating, alpha particles have a low penetrating ability. Beta-minus particles have a medium penetrating ability. They can travel about 2 m in air or about 1-2 mm in human tissue before they slow down too much to cause ionizations.
This Geiger counter is detecting the beta-minus particles being emitted by the tiny speck of strontium-90 that’s in the small steel container that I’m holding. Unlike alpha particles, the beta-minus particles can go straight through the paper, but they are stopped by a thin piece of metal.
Gamma rays have a very high penetrating ability. They can travel 10’s of metres in air and several metres in water. Most of the gamma rays coming from the tiny speck of cobalt-60 in the steel housing pass straight through the thin lead sheeting, and even a really thick lead block doesn’t quite stop all of them.
Finally, in terms of their ability to ionize atoms, alpha particles have a very high ability, beta-minus particles a medium ability, and gamma rays a low ability.
By the way, while filming these scenes, my hands received a tiny tiny dose of nuclear radiation, but it wasn’t anywhere near enough to cause any concern. However, if I did it day after day, it wouldn’t be a good idea.
So, gamma rays are less ionizing than beta-minus particles, but they have a greater penetrating ability. It turns out that this makes beta-minus particles and gamma rays about as damaging as each other if they’re being emitted inside your body. Alpha particles don’t go far, but in the short distance that they do travel at high speeds, if they are emitted in your body, they do about 20 times as much damage as the other two. This is because they’re heavy and have that double positive charge.
So, let’s now look at a few examples of how we put beta-minus and gamma radiation to good use in medicine and in industry.
Part E: Putting Beta-Minus and Gamma Radiation to Work


 In this factory, the gamma rays being produced by cobalt-60 are being used to sterilize medical equipment and lots of other things. To sterilize something means to kill all the germs on it. Small pellets of cobalt-60 are placed onto a frame that is about three metres wide. Boxes containing bandages, syringes, latex gloves and all manner of medical equipment are carried by conveyor belts past the cobalt-60 and the gamma rays produced by the cobalt-60 kill any germs that might have found their way into the packaging. The high penetrating ability of the gamma rays means that the equipment doesn’t have to be unboxed. Of course, they don’t do one box at a time, it’s a continuous process. The technique of hitting something with radiation is called irradiation. The boxes are being irradiated. Surrounding the irradiation room are thick concrete walls, so no gamma rays come anywhere near any workers who process the boxes.
In this factory, the gamma rays being produced by cobalt-60 are being used to sterilize medical equipment and lots of other things. To sterilize something means to kill all the germs on it. Small pellets of cobalt-60 are placed onto a frame that is about three metres wide. Boxes containing bandages, syringes, latex gloves and all manner of medical equipment are carried by conveyor belts past the cobalt-60 and the gamma rays produced by the cobalt-60 kill any germs that might have found their way into the packaging. The high penetrating ability of the gamma rays means that the equipment doesn’t have to be unboxed. Of course, they don’t do one box at a time, it’s a continuous process. The technique of hitting something with radiation is called irradiation. The boxes are being irradiated. Surrounding the irradiation room are thick concrete walls, so no gamma rays come anywhere near any workers who process the boxes.
If anything goes wrong, the whole frame drops down into an 8-metre-deep pool of water, which completely absorbs the gamma rays and so workers can come in and fix things or carry out maintenance.
So, we can be very confident that any kind of medical equipment that we open is free of any germs, thanks to facilities like the one we’ve just seen.

 Now in hospitals, different radioisotopes of iodine are used to diagnose and to treat thyroid cancer and other thyroid conditions.
Now in hospitals, different radioisotopes of iodine are used to diagnose and to treat thyroid cancer and other thyroid conditions.
The thyroid is a small gland in our neck that produces various hormones that regulate energy production and protein production in our cells. Two of the hormones that it produces contain iodine atoms. A lot of the tiny amounts of iodine that we eat in our diet ends up in the thyroid gland where it is used to make the hormones.
Occasionally, problems occur in the thyroid which can lead to too much hormone production or to not enough hormone production. Cancer is often responsible.

 To try to diagnose the thyroid problem, patients can be given a very small amount of radioactive iodine-123, which is a gamma emitter. The iodine is taken up by the thyroid and the emitted gamma rays stream out of it. A gamma-ray detector placed above the patient’s thyroid records an image of the thyroid. Doctors can tell by the shape and the darkness of the image what the problem is.
To try to diagnose the thyroid problem, patients can be given a very small amount of radioactive iodine-123, which is a gamma emitter. The iodine is taken up by the thyroid and the emitted gamma rays stream out of it. A gamma-ray detector placed above the patient’s thyroid records an image of the thyroid. Doctors can tell by the shape and the darkness of the image what the problem is.
 Medical imaging like this takes advantage of the fact that gamma rays are highly penetrating.
Medical imaging like this takes advantage of the fact that gamma rays are highly penetrating.
If thyroid cancer is diagnosed, doctors will sometimes decide (depending on the size of the cancer and other factors) that the best course of action is to use nuclear radiation to destroy it. To that end, they use iodine-131, which is a beta-minus and gamma emitter.
It is usually given in tablet form. The iodine-131 is absorbed by the thyroid, and the beta-minus particles that are emitted inside the thyroid by the iodine-131 kill the cancer cells. The vast majority of the gamma rays being produced just pass out of the body.
 Remember, cancer cells are cells that are rapidly multiplying in an out-of-control way. These cells absorb more iodine and are more susceptible to ionizing radiation than normal healthy cells are. Using nuclear radiation to kill cancer cells is called radiotherapy. The beta-minus particles travel at most only about 2 mm, before slowing down so much that they can’t do any more damage. Tissue outside the thyroid receives very little radiation. Some types of cancer are best treated with certain chemicals, and this process is called chemotherapy.
Remember, cancer cells are cells that are rapidly multiplying in an out-of-control way. These cells absorb more iodine and are more susceptible to ionizing radiation than normal healthy cells are. Using nuclear radiation to kill cancer cells is called radiotherapy. The beta-minus particles travel at most only about 2 mm, before slowing down so much that they can’t do any more damage. Tissue outside the thyroid receives very little radiation. Some types of cancer are best treated with certain chemicals, and this process is called chemotherapy.
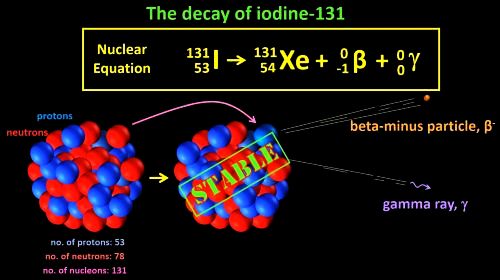
Let’s write the nuclear equation for the decay of iodine-131. Iodine-131 can be written in atomic notation as 131 53 I. A beta-minus particle and a gamma ray are emitted and we can add them to the equation. So, what is the daughter nucleus left behind? Well, one of the neutrons has turned into a proton (and an electron, which is emitted) so the daughter nucleus ends up with one more proton, that is 54 protons. This makes it a Xenon atom (or a “Zenon” atom if you prefer that pronunciation). The mass number stays the same, 131. Now xenon-131 is stable and so no more radiation is produced. It’s also non-toxic and the body slowly gets rid of it.
131
53I
— > 131
54Xe
+ 0
-1![]()
+ 0
0![]()
 And that brings us to the end of the lesson. Now so far, we’ve covered alpha, beta-minus, and gamma decay. In our next lesson, we’re going to look at beta-plus decay which involves the creation of electrons with a positive charge. The universe is amazing. See you then.
And that brings us to the end of the lesson. Now so far, we’ve covered alpha, beta-minus, and gamma decay. In our next lesson, we’re going to look at beta-plus decay which involves the creation of electrons with a positive charge. The universe is amazing. See you then.
CREDITS:
“Cancer is not one disease” by Garvan Institute of Medical Research. https://youtu.be/BlajAw8exg4. Creative Commons License.
“Cherenkov Radiation in 60 seconds” by IAEAvideo. https://youtu.be/4hijBTgrvjY. Creative Commons License.
“How radiopharmaceuticals help diagnose cancer and cardiovascular disease” by IAEA. https://youtu.be/mQjCTTKWOFU. Creative Commons License.
Footage of Gamma Irradiation © BGS Beta-Gamma-Service. Used with Permission. See “BGS Beta-Gamma-Service _ Using gamma rays to destroy germs_ How radiation sterilization works” by BGS Beta Gamma Service. https://youtu.be/hblMTH09KJQ
“The IAEA and Health_ Nuclear Medicine – Sri Lanka” by IAEAvideo. https://youtu.be/UvIhXa0_4N4. Creative Commons License.
Special thanks to the University of Melbourne School of Physics.



